Battery cells with internal reference electrodes
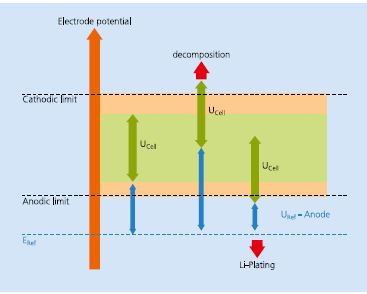
To increase the safety of Li-ion batteries, knowledge of the actual electrode potential is desirable. In a 2-electrode system, where only the voltage difference between the anode and cathode is known, one of the electrode potentials could still be outside its safe limit. In this case, a normal voltage difference between the electrodes masks the violation of the safe potential limit of one electrode, as shown in Figure 1.
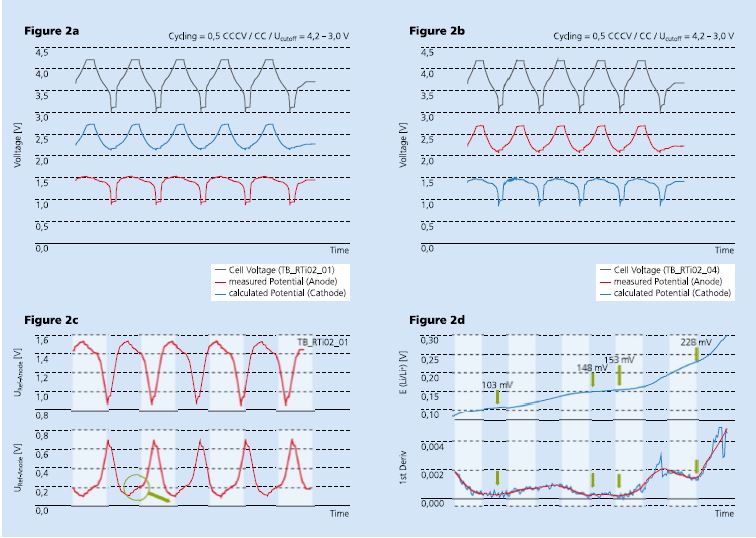
In a system with a reference electrode, the electrode potentials can be directly monitored and the battery management system is able to terminate battery operation. This can prevent common problems of lithium-ion batteries, such as lithium dendrite formation or the occurrence of oxidation processes at the cathode.
 Fraunhofer Institute for Silicon Technology
Fraunhofer Institute for Silicon Technology